Get Healthy!
Results for search "Food &, Drug Administration".
Health News Results - 419
The U.S. Food and Drug Administration’s (FDA) chief says the agency will begin offering bonuses to drug reviewers who complete their work ahead of schedule.
Dr. Marty Makary described the effort as a pilot program during a staff meeting last week. The first quarterly bonus payments could begin going out in Au...
- HealthDay Staff HealthDay Reporter
- |
- March 2, 2026
- |
- Full Page
U.S. health officials are proposing a new way to develop and approve custom-made treatments for people with rare and hard-to-treat conditions.
The U.S. Food and Drug Administration (FDA) just released a draft of guidelines that would create a special pathway for therapies designed for just a small number of people. Drug companies often avoid these, because they are considered unprofitable...
- I. Edwards HealthDay Reporter
- |
- February 24, 2026
- |
- Full Page
In a major blow to vaccine development, the U.S. Food and Drug Administration (FDA) said it will not review Moderna’s application for the first mRNA-based flu shot.
Dr. Vinay Prasad, the nation’s top vaccine regulator, told the company it lacked an "adequate and well-controlled" study, Moderna<...
- Carole Tanzer Miller HealthDay Reporter
- |
- February 12, 2026
- |
- Full Page
The U.S. Food and Drug Administration (FDA) is taking a fresh look at the safety of a chemical preservative found in many packaged foods.
The agency announced it has launched a full review to decide whether butylated hydroxyanisole, or BHA, is still safe to use in food and food packaging based on the latest science.
As part of that process, the FDA is asking the public to submit new...
- I. Edwards HealthDay Reporter
- |
- February 11, 2026
- |
- Full Page
Olympia Provisions has recalled about 1,930 pounds of ready-to-eat holiday sausage.
The recalled meat is wrapped and vacuum-sealed in 16-ounce clear pouches and labeled “OLYMPIA PROVISIONS UNCURED HOLIDAY KIELBASA.”
The U.S. Department of Agriculture’s Food Safety and Inspection Service (FSIS)
The U.S. Food and Drug Administration (FDA) is weighing a change that could make warning labels on dietary supplements appear less often on packaging.
Unlike prescription drugs, dietary supplements are not reviewed by the FDA for safety or effectiveness before they are sold.
Instead, a 1994 federal law requires companies to include a disclaimer whenever they make health claims, such...
- I. Edwards HealthDay Reporter
- |
- December 19, 2025
- |
- Full Page
THURSDAY, Dec. 18, 2025 (HealthDay News — The U.S. Food and Drug Administration (FDA) approved a new safety warning for Depo-Provera, a widely used birth control shot made by Pfizer, alerting patients to a possible risk for a type of brain tumor called meningioma.
The agency signed off last w...
- I. Edwards HealthDay Reporter
- |
- December 18, 2025
- |
- Full Page
Americans may soon have access to a new sunscreen ingredient already used around the world.
The U.S. Food and Drug Administration (FDA) announced Dec. 11 that it is reviewing a proposal to allow bemotrizinol in sunscreens sold in the United States.
The ingr...
- I. Edwards HealthDay Reporter
- |
- December 12, 2025
- |
- Full Page
An at-home device that sends a gentle electrical current to the brain to help treat depression has been cleared by the U.S. Food and Drug Administration (FDA).
Experts say the move could expand access to care for many folks.
The prescription headset, made by Sweden-based <...
- I. Edwards HealthDay Reporter
- |
- December 12, 2025
- |
- Full Page
The U.S. Food and Drug Administration (FDA) is facing even more leadership changes as drug regulator Dr. Richard Pazdur prepares to retire at the end of the month, the agency confirmed this week.
Pazdur, who has worked at the FDA for 26 years, told senior leaders on Tuesday that he plans to step down, just weeks a...
- I. Edwards HealthDay Reporter
- |
- December 4, 2025
- |
- Full Page
The U.S. Food and Drug Administration (FDA) is tightening restrictions on a gene therapy used to treat Duchenne muscular dystrophy after two teenagers died from liver failure linked to the medication.
The FDA decision limits the use of Elevidys, made by Sarepta Therapeutics, to boys who are 4 years an...
- I. Edwards HealthDay Reporter
- |
- November 17, 2025
- |
- Full Page
The U.S. Food and Drug Administration (FDA) has appointed one of its most respected cancer drug regulators to lead the agency’s main division for approving new drugs.
The appointment of Dr. Richard Pazdur comes after a turbulent year with hundreds of staff departures within the agency.
Pazdur, who has ...
- I. Edwards HealthDay Reporter
- |
- November 13, 2025
- |
- Full Page
The U.S. Food and Drug Administration (FDA) is eliminating the prominent "black box" warnings on many hormone replacement therapy (HRT) medications, signaling a major shift in how the treatment is viewed for menopausal women.
The decision affects products containing estrogen or progestogen, alone or combined, that are prescribed to treat troublesome menopause symptoms like hot flashes, mo...
- Deanna Neff HealthDay Reporter
- |
- November 12, 2025
- |
- Full Page
The head of the U.S. Food and Drug Administration’s (FDA) drug division has resigned while under internal investigation, saying he was pushed out after raising concerns about how the agency planned to fast-track some new drugs.
Dr. George Tidmarsh, who joined the FDA in July, stepped down Sunday, The New York T...
- I. Edwards HealthDay Reporter
- |
- November 4, 2025
- |
- Full Page
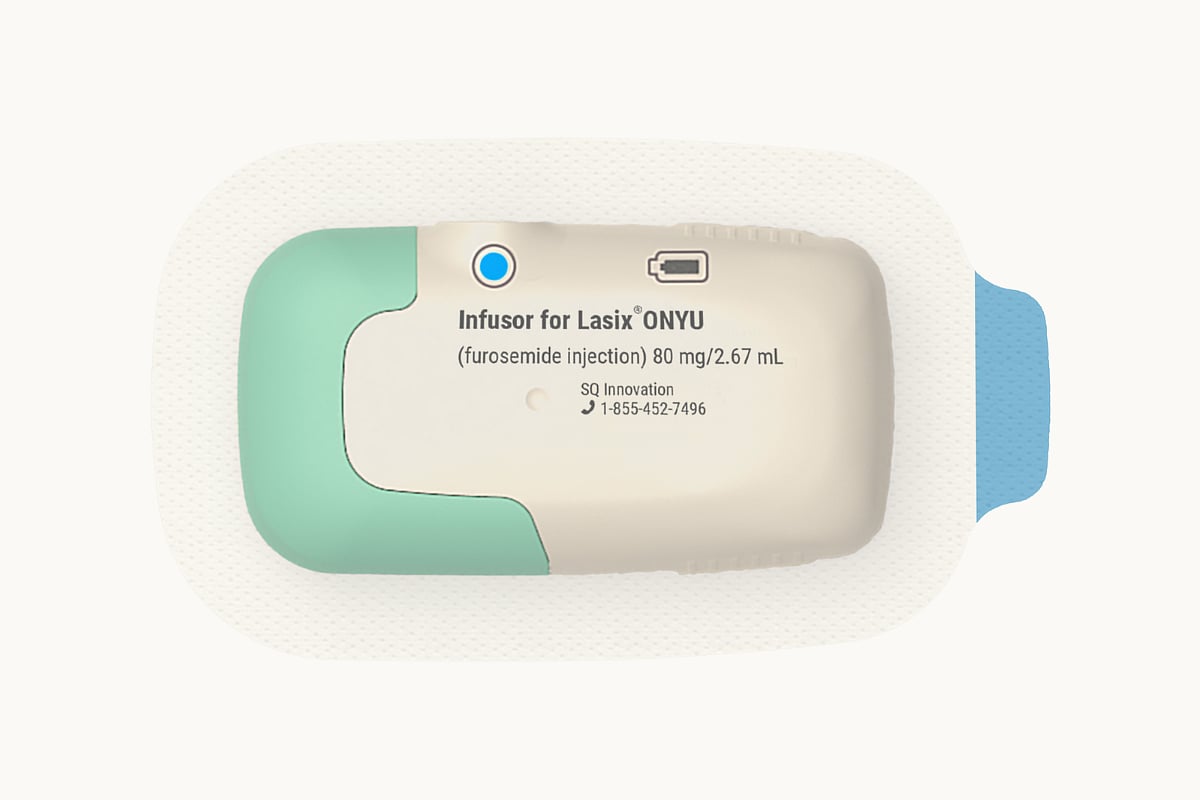
A new at-home version of a common heart failure drug could make treatment easier for millions of Americans.
The U.S. Food and Drug Administration (FDA) has approved Lasix ONYU (furosemide injection), a new drug-device combination developed by SQ Innovation, Inc., for treating edema caused by chronic
For the first time, people can get their annual flu vaccine without leaving the comfort of home.
FluMist, a nasal spray flu vaccine made by AstraZeneca, is now available for at-home use through a service called FluMist Home, the company announced.
The U.S. Food and Drug Administration (FDA) first approved FluMist in 2003 for use in doctors&rsqu...
- I. Edwards HealthDay Reporter
- |
- August 19, 2025
- |
- Full Page
The U.S. Food and Drug Administration (FDA)’s top vaccine regulator is returning to his post less than two weeks after the White House had him ousted.
Dr. Vinay Prasad will again head the FDA’s Center for Biologics...
- I. Edwards HealthDay Reporter
- |
- August 11, 2025
- |
- Full Page
The U.S. Food and Drug Administration’s (FDA) top vaccine official is stepping down after just three months in a role that upset drug companies, patient groups and some political leaders.
Dr. Vinay Prasad will leave the agency to “spend more time with his family,” a spokesperson for the U.S. Department of Health and Human Service...
- HealthDay Reporter
- I. Edwards
- |
- July 31, 2025
- |
- Full Page
Ritz peanut butter cracker sandwiches are being recalled due to the possible presence of undeclared peanuts, a major allergen.
The affected products contain individually wrapped packs that may have been mislabeled as cheese sandwiches instead of peanut butter ones, according to Newsweek. The outer cartons were labeled correctly and indicated the presence of peanuts.
...- HealthDay Reporter
- Denise Mann
- |
- July 25, 2025
- |
- Full Page
The U.S. Food and Drug Administration (FDA) has upgraded a recall of a commonly prescribed thyroid medication due to what it described as "subpotent" active ingredients.
The recall of more than 160,000 bottles of levothyroxine sodium, which went into effect June 20, was upgraded to a Class II recall on July 23. Class II recalls occur ...
- HealthDay Reporter
- Denise Mann
- |
- July 24, 2025
- |
- Full Page
WEDNESDAY, July 23, 2025 (HealthDay News) —Two types of tuna sold in seven states are being recalled due to listeria concerns.
The first recall affects tuna salad and ready-to-eat foods containing tuna salad from Beaverton, Oregon-based Reser's Fine Foods. The product...
- HealthDay Reporter
- Denise Mann
- |
- July 23, 2025
- |
- Full Page
More than 67,000 cases of Power Stick deodorant have been recalled due to an undisclosed manufacturing issue.
The recalled deodorants, made by A.P. Deauville of Easton, Pa., did not fully comply with federal product safety standards, according to the U.S. Food and Drug Administration (FDA).
In issuing
Thousands of health workers lost their jobs this week after a U.S. Supreme Court ruling cleared the way for the Trump administration to move forward with major staffing cuts.
On Monday, the U.S. Department of Health and Human Services (HHS) finalized 10,000 layoffs across federal h...
- HealthDay Reporter
- I. Edwards
- |
- July 16, 2025
- |
- Full Page
The U.S. Food and Drug Administration (FDA) says it plans to use artificial intelligence (AI) to help speed the approval of new drugs and medical devices.
That's one of several priorities federal officials detailed June 10 in JAMA.
They said AI could help shorten review times, speeding delivery of treat...
- HealthDay Reporter
- I. Edwards
- |
- June 11, 2025
- |
- Full Page
Salmonella-tainted tomatoes in three southern states could cause severe illness or even death, the U.S. Food and Drug Administration (FDA) warns.
The FDA has updated an ongoing recall of tomatoes distributed in Georgia, North Carolina and South Carolina to Class I — its highest warning level, The New York Times reported.
A Class I recall means there is a reasonable ch...
- HealthDay Reporter
- I. Edwards
- |
- June 2, 2025
- |
- Full Page
Publix is recalling one of its popular GreenWise baby food pouches because it may contain lead, the company said this week.
The recalled product is the Pear, Kiwi, Spinach & Pea Baby Food pouch.
The supermarket chain said it found the issue through routine test...
- HealthDay Reporter
- I. Edwards
- |
- May 20, 2025
- |
- Full Page
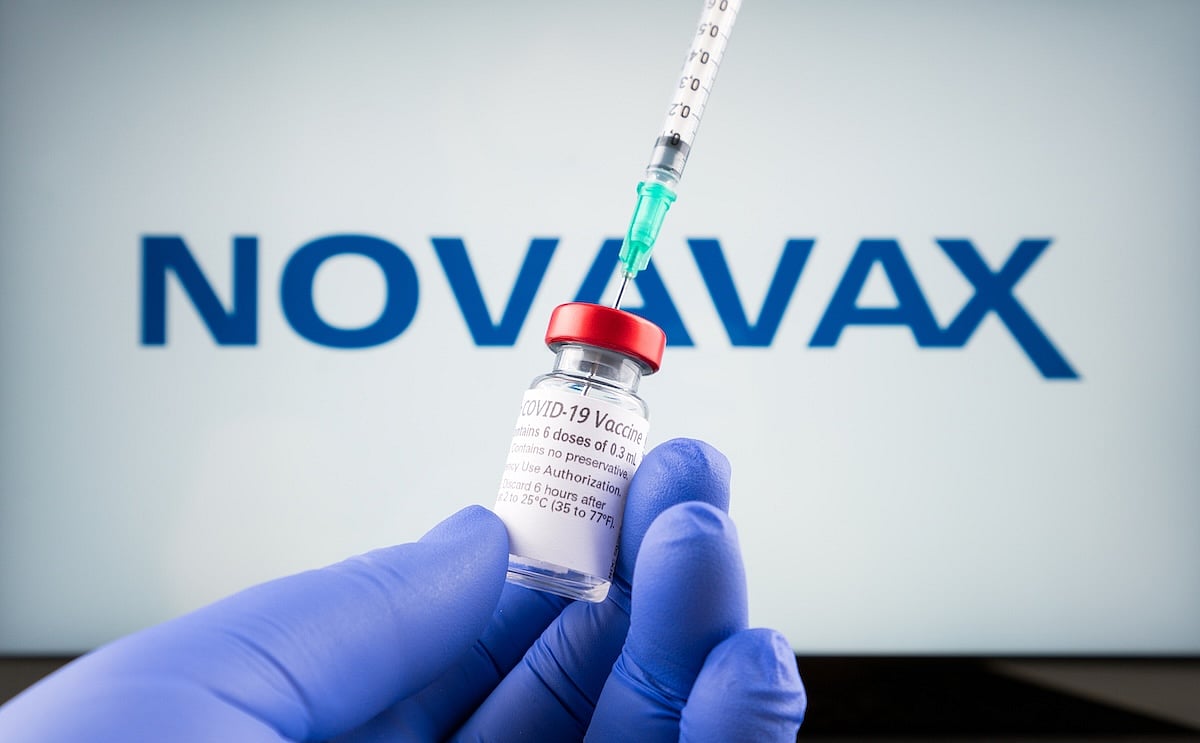
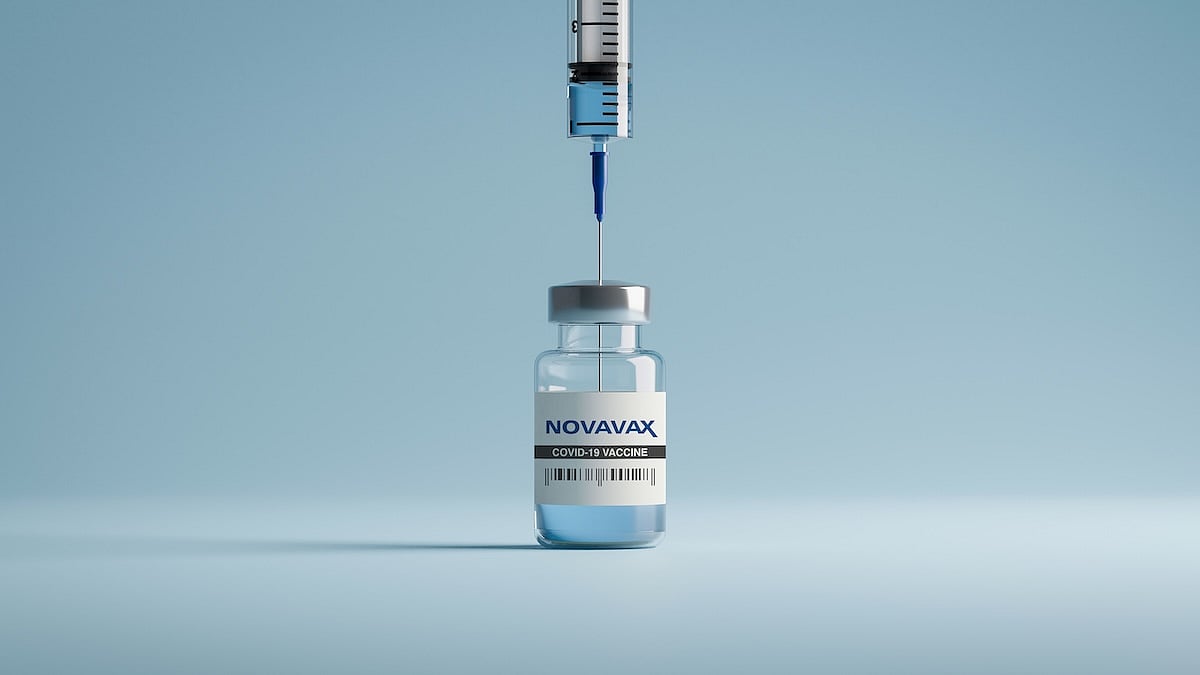
The U.S. Food and Drug Administration (FDA) has granted full approval to Novavax’s COVID-19 vaccine, but only for certain people.
The vaccine is now approved for adults ages 65 and older, or for people ages 12 to 64 who have at least one health condition that puts them at higher risk of serious illness from
Women now have a new way to check their risk for cervical cancer -- from the comfort of their own home.
The U.S. Food and Drug Administration (FDA) has approved the Teal Wand, an at-home test that screens for human papillomavirus (HPV), the virus tha...
- HealthDay Reporter
- I. Edwards
- |
- May 12, 2025
- |
- Full Page
The U.S. Food and Drug Administration (FDA) is warning consumers and tattoo artists that two tattoo inks have tested positive for harmful bacteria and could lead to serious infections.
The affected products are:
Sacred Tattoo Ink, Raven Black (CI# 77266; Lot#: RB0624, Best Before: June 28, 2027)
Sacred Tattoo Ink, Sunny Daze (CI# 21095; Lot#: SD1124, Best Befo...
- HealthDay Reporter
- I. Edwards
- |
- May 9, 2025
- |
- Full Page
The U.S. Food and Drug Administration (FDA) has chosen Dr. Vinay Prasad, a professor at the University of California-San Francisco, to lead its Center for Biologics Evaluation and Research.
The division oversees vaccines and biologic medicines, including gene therapies, CNN reported.
Prasad is a hematologist-oncologist, a spec...
- HealthDay Reporter
- I. Edwards
- |
- May 7, 2025
- |
- Full Page
The official in charge of federal food and drug safety inspections will retire May 14.
Michael Rogers, associate commissioner for inspections and investigations at the U.S. Food and Drug Administration (FDA), announced his decisio...
- HealthDay Reporter
- I. Edwards
- |
- May 7, 2025
- |
- Full Page
The head of the U.S. Food and Drug Administration (FDA) reiterated Tuesday that the agency is applying a more skeptical approach to this year’s round of COVID-19 vaccine boosters.
Companies applying for approval of COVID boosters are being encouraged to use “gold standard science,” ...
- HealthDay Reporter
- Dennis Thompson
- |
- May 6, 2025
- |
- Full Page
Dr. Marty Makary had just finished his last surgery at Johns Hopkins when he stepped into one of the most powerful roles in American public health.
Now, as the new commissioner of the U.S. Food and Drug Administration (FDA), he's wasting no time charting a new course for the...
- HealthDay Reporter
- I. Edwards
- |
- May 1, 2025
- |
- Full Page
A quick spray of medication might seem like an easy way to get thicker hair, but some folks say one sold online has left them battling sexual side effects, depression and even thoughts of suicide.
Now, the U.S. Food and Drug Administration (FDA) is warning the public about the r...
- HealthDay Reporter
- I. Edwards
- |
- April 24, 2025
- |
- Full Page
The U.S. Food and Drug Administration (FDA) may soon hand off routine food safety inspections to state and local officials, multiple federal health officials told CBS News.
These changes are not final and could require approval and funding from Congress. Some FDA employees have been discussing a handoff for years. The goal is to free up federal staff for higher priotiry or forei...
- HealthDay Reporter
- I. Edwards
- |
- April 21, 2025
- |
- Full Page
Weeks after ordering staff back to the office, the U.S. Food and Drug Administration (FDA) is now letting some employees work from home again.
The move follows major staff cuts and resignations that threaten the agency’s ability to approve new medicines among other basic functions, The Associated Press reported.
An internal email shared with staff on Tuesday said FDA...
- HealthDay Reporter
- I. Edwards
- |
- April 10, 2025
- |
- Full Page
The U.S. Food and Drug Administration’s (FDA) top tobacco regulator, Brian King, has been placed on leave as part of a large wave of cuts across federal health agencies.
King, who led the FDA's tobacco control efforts, told his staff Tuesday that he was removed with “a heavy heart and profound disappointment.”
“If you make it virtually impossible to cre...
- HealthDay Reporter
- I. Edwards
- |
- April 2, 2025
- |
- Full Page
A top vaccine official at the U.S. Food and Drug Administration (FDA) is stepping down, warning that vaccine misinformation is coloring the country’s top health decisions.
Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said he will resign and retire by April 5.
...
- HealthDay Reporter
- I. Edwards
- |
- March 31, 2025
- |
- Full Page
Health officials are warning about a rise in injuries linked to the misuse of nitrous oxide, aka laughing gas.
The gas, which is used medically as a sedative and in whipped cream cans, is now being sold in small, flavored canisters with names such as Cosmic Gas, Galaxy Gas and MassGass.
On Friday, the U.S. Food and Drug Administration (FDA) issued a warning that inhaling nitrous oxi...
- HealthDay Reporter
- I. Edwards
- |
- March 17, 2025
- |
- Full Page
A major recall of canned tuna sold in dozens of states has been issued amid concerns that a packaging defect could cause “a potentially fatal form of food poisoning,” the manufacturer said in a
- HealthDay Reporter
- India Edwards
- |
- February 12, 2025
- |
- Full Page
A federal judge has ordered the U.S. Centers for Disease Control and Prevention (CDC) and Food and Drug Administration (FDA) to restore access to public health websites that were removed or modified in response to a Trump administration executive order on gender.
The ord...
- HealthDay Reporter
- India Edwards
- |
- February 12, 2025
- |
- Full Page
A rather historic U.S. Food and Drug Administration (FDA) proposal aims to make cigarettes and other tobacco products far less addictive by reducing their nicotine content.
The move could help millions of Americans quit smoking while preventing many more from becoming addic...
- HealthDay Reporter
- India Edwards
- |
- January 16, 2025
- |
- Full Page
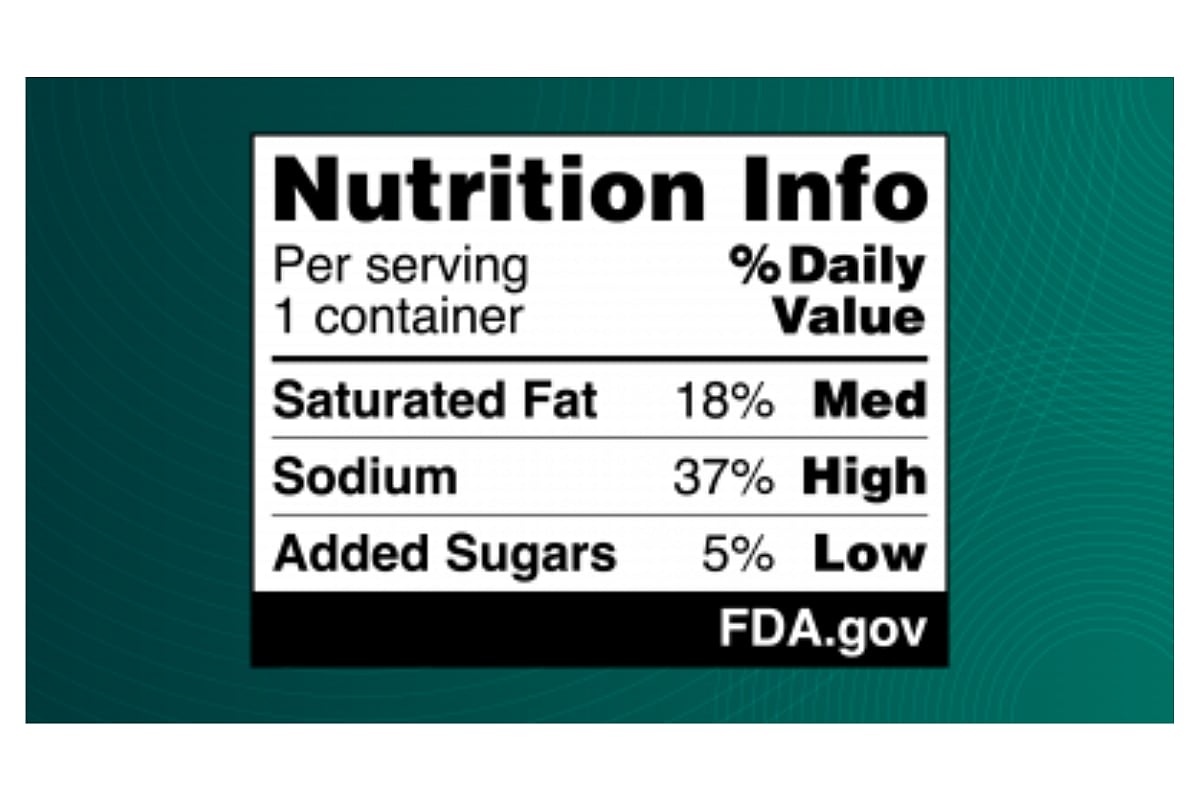
Grabbing a quick snack might soon come with a little extra clarity.
The U.S. Food and Drug Administration (FDA) has proposed a new rule requiring bold, easy-to-read nutrition labels on the front of food and beverage packages.
These labels, which would highlight content of sugar, salt, and saturated fat, aim to make it easier for shoppers to make healthier choices in the grocery ais...
- HealthDay Reporter
- India Edwards
- |
- January 15, 2025
- |
- Full Page
The U.S. Food and Drug Association (FDA) released the first-ever guidelines for levels of lead in processed baby foods this week. However, many health and safety advocates say they are not satisfied with the guidance.
Under the
The U.S. Food and Drug Administration (FDA) has proposed a new rule to require standardized testing of talc-containing cosmetics for asbestos, a known carcinogen linked to serious illnesses such as lung and ovarian cancers.
According to an FDA